Abstract
Background: Myeloablative conditioning can be given safely to older patients by simply administering busulfan (Bu) over a longer period (fractionated (f)-Bu) with fludarabine (Flu) than the standard four-day regimen. (Popat et al Lancet Haematology 2018). We added thiotepa (Thio) to this f-Bu/Flu regimen in patients with haploidentical (haplo) donors to promote engraftment and to reduce relapse. With encouraging results, Thio was then added in matched unrelated (MUD) and sibling (MSD) donor recipients also. However, the optimal doses of Thio and Bu were undefined. To further optimize the regimen, we tested two different f-Bu/Flu/Thio regimens in parallel prospective cohorts: (a) higher dose Bu with lower dose Thio, and (b) lower dose Bu with a higher dose Thio. Patients were enrolled on these cohorts based on age and co-morbidity index (HCT-CI). Herein, we report on the safety and preliminary efficacy of these regimens (NCT02861417).
Methods: Patients <60 years with HCT-CI <3, or >60 years with HCT-CI <2 received IV f-Bu to target an area under the concentration vs time curve (AUC) of 20,000 ± 12% μmol.min with Thio 2.5 mg/kg (Bu20K/Thio2.5; Cohort 1). Patients <60 years with an HCT-CI >3, or >60 years with HCT-CI >2, or >70 years irrespective of HCT-CI received f-BU to target AUC of 16,000 with Thio 5mg/kg (Bu16K/Thio5; Cohort 2). In both cohorts, the first two doses of Bu (80 mg/m2 IV each) were given as outpatient on days -20 and -13. The last four Bu doses were given as inpatient following each dose of Flu 40 mg/m2 on days -6 through -3. Thio was given on day -7. GVHD prophylaxis was post-transplant cyclophosphamide (PTCy) 50mg/kg on days 3 and 4, tacrolimus and MMF.
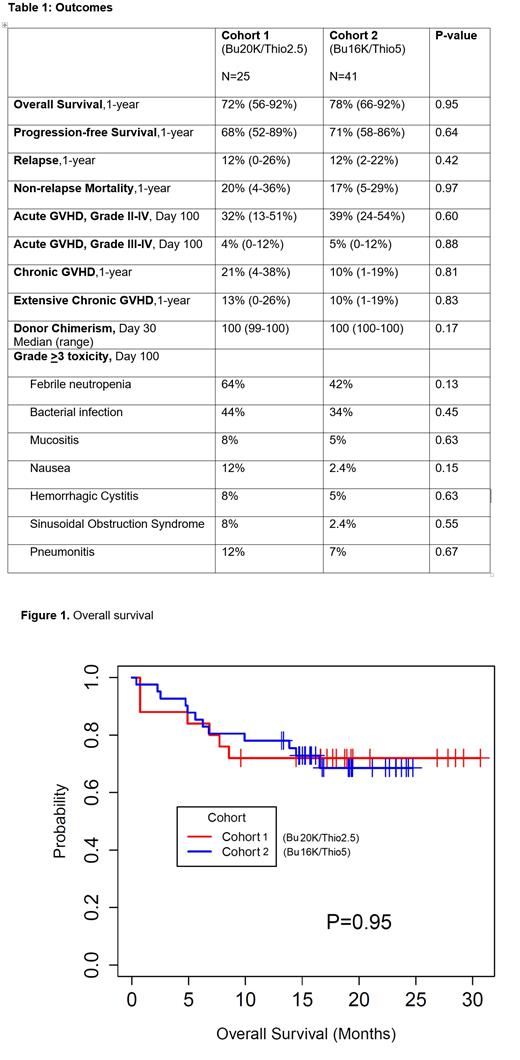
Results: 66 patients were enrolled - 25 in cohort 1 and 41 in cohort 2, with a median age 60 years (range, 22-69) vs 62 years (range, 30-74), respectively (P=0.14). Diagnoses were AML/MDS (64% vs 54%), CML/MPD (24% vs 22%), ALL (12% vs 22%) and 1 myeloma; P=0.24. Most had intermediate disease risk index (80% vs 73%, P=0.57); median HCT-CI was 1 (range, 0-6) vs 3 (range, 0-9); P<0.0001. Donor was haplo (60% vs 19%), MUD (24% vs 54%) or MSD (16% vs 27%); P=0.002; BM graft was used in 56% vs 27%; p=0.02, respectively, in cohorts 1 and 2. The median follow-up among survivors was 18.7 months.
Primary graft failure occurred in 1 patient in cohort 1 and none in cohort 2. The median time to neutrophil engraftment was 17.5 days (range, 14-26) vs 18 days (range, 12-26); P=0.12, respectively; platelet engraftment > 20K was 30 days in both groups; P=0.75. Median donor chimerism at day 30 was 100% in both groups. The incidence of acute GVHD grade III-IV was 4% vs 5%; P=0.88 at day 100; extensive chronic GVHD at 1 year was 13% vs 10%; P=0.83. At 1-year overall survival was 72% vs 78%; P=0.95; relapse was 12% each; non-relapse mortality was 20% vs 17%; P=0.97 [Table 1, Fig 1]. Common grade >3 toxicities were neutropenic fever (64% vs 42%; P=0.13), bacterial infection (44% vs 34%; P=0.45), nausea (12% vs 2%; P=0.15), mucositis (8% vs 5%; P=0.63), pneumonitis (12% vs 7%; P=0.67), hemorrhagic cystitis (8% vs 5%) and sinusoidal obstructive syndrome (8% vs 2.4%).
Conclusion: In this population of older patients and/or high co-morbidities, both myeloablative regimens with f-Bu/Flu with thiotepa and PTCy were well tolerated and led to low NRM, low risk of relapse and encouraging survival. Although both regimens led to similar outcomes, the f-Bu/Flu/Thio regimen with Bu16K and thiotepa 5 mg/kg dose may be preferred as this was used in patients who were older and/or had high co-morbidities.
Popat: Bayer: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Mehta: CSLBehring: Research Funding; Kadmon: Research Funding; Syndax: Research Funding; Incyte: Research Funding. Hosing: Nkarta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Rezvani: Caribou: Other: Scientific Advisory Board; Virogin: Other: Scientific Advisory Board ; AvengeBio: Other: Scientific Advisory Board ; GSK: Other: Scientific Advisory Board ; Bayer: Other: Scientific Advisory Board ; Navan Technologies: Other: Scientific Advisory Board; GemoAb: Other: Scientific Advisory Board ; Takeda: Other: License agreement and research agreement, Patents & Royalties; Pharmacyclics: Other: Educational grant, Research Funding; Affimed: Other: License agreement and research agreement; education grant, Patents & Royalties, Research Funding. Qazilbash: Oncopeptides: Other: Advisory Board; Janssen: Research Funding; Bristol-Myers Squibb: Other: Advisory Board; NexImmune: Research Funding; Amgen: Research Funding; Angiocrine: Research Funding; Biolline: Research Funding. Shpall: Affimed: Patents & Royalties; Adaptimmune: Consultancy; Novartis: Consultancy; Magenta: Consultancy; Magenta: Honoraria; Axio: Consultancy; Takeda: Patents & Royalties; Navan: Consultancy; Novartis: Honoraria; Bayer HealthCare Pharmaceuticals: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal